Biologically Active Cyclic Peptide Natural Products
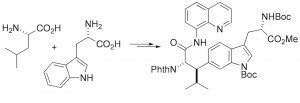
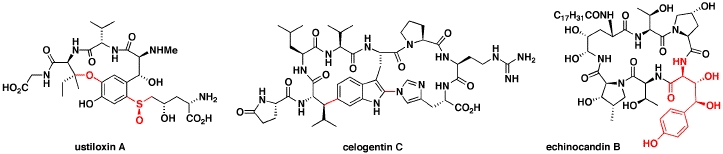
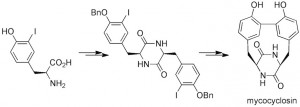
Current efforts are being directed towards the synthesis of several complex cyclic peptide natural products. The ustiloxins & celogentins are tubulin-binding cyclic peptides that have shown potent in vitro anti-tumour activity. The echinocandins and microsclerodermins are antifungal cyclic peptides that contain several poly-hydroxylated amino acids, with several now in clinical use for the treatment of fungal infections. The general synthetic strategy involves developing methods for the stereocontrolled synthesis of the highly functionalised amino acid residues present in these peptides, followed by the development of procedures for the assembly of the amino acid components into the final cyclic peptides. We have recently prepared the dityrosine-containing cyclic peptide natural product mycocyclosin, combining our interests in dityrosine crosslinks and peptide natural products!
Recent Publications
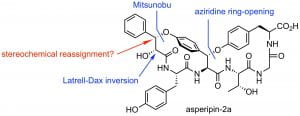
S. Shabani, J. M. White, C. A. Hutton, “Total Synthesis of the Putative Structure of Asperipin-2a and Stereochemical Reassignment,” Org. Lett. 2020, 22, 7730–7734.
S. Shabani, C. A. Hutton, “Total Synthesis of Seongsanamide B”, Org. Lett., 2020, 22, 4557–4561.
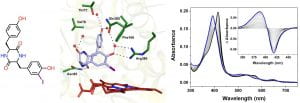
S. Rajput, K. J. McLean, H. Poddar, I. R. Selvam, G. Nagalingam, J. A. Triccas, C. W. Levy, A. W. Munro, C. A Hutton, “Structure–activity relationships of cyclo(L-tyrosyl-L-tyrosine) derivatives binding to Mycobacterium tuberculosis CYP121: iodinated analogues promote shift to high-spin adduct,” J. Med. Chem. 2019, 62, 9792–9805.
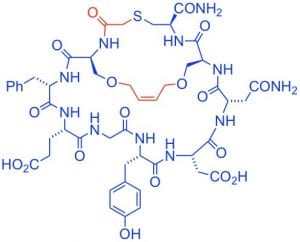
S. Shabani, J. M. White, C. A. Hutton, “Synthesis of the C-Terminal Macrocycle of Asperipin-2a,” Org. Lett., 2019, 21, 1877–1880.
V. J. Thombare, C. A. Hutton “Bridged Bicyclic Peptides: Structure and Function,” Peptide Science, 2018, 110, e24057.
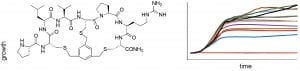
V. J. Thombare, J. A. Holden, S. Pal, E. C. Reynolds, A. Chattopadhyay, N. M. O’Brien-Simpson, C. A. Hutton “Antimicrobial activity of simplified mimics of celogentin C,” Tetrahedron, 2018, 74, 1288–1293.
A. L. Brown, N. L. Fifer, Q. I. Churches, P. W. H. Chan, S. B. Cohen, C. A. Hutton, “Total Synthesis of Ustiloxin D,” in Strategies and Tactics in Organic Synthesis, M. Harmata, ed, Academic Press, 2016, 12, Chapter 6, pp. 169–191. ISBN: 9780081007563 eISBN: 9780081007624.
A. L. Brown, Q. I. Churches, C. A. Hutton, “Total Synthesis of Ustiloxin D Utilizing an Ammonia–Ugi Reaction,” J. Org. Chem., 2015, 80, 9831–9837.
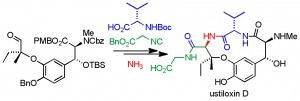
J. R. Cochrane, J. M. White, U. Wille and C. A. Hutton, “Total Synthesis of Mycocyclosin,” Org. Lett. 2012, 14, 2402–2405.
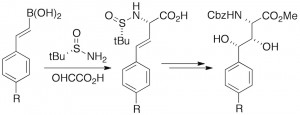
Q. I. Churches, J. M. White, C. A. Hutton, “Synthesis of β,γ-Dihydroxyhomotyrosines by a Tandem Petasis–Asymmetric Dihydroxylation Approach,” Org. Lett. 2011, 13, 2900–2903.
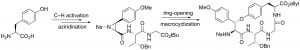
B. T. Y. Li, J. M. White, C. A. Hutton, “Synthesis of the Leu–Trp component of the celogentin family of cyclic peptides through a C–H activation–cross-coupling strategy,” Aust. J. Chem. 2010, 63, 438–444.