Exploiting Thioamide Reactivity in Peptide Synthesis
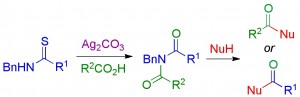
We have developed a new method for peptide synthesis through the Ag(I)-promoted coupling of thioamides with carboxylic acids. This process generates an isoimide, which can undergo a 1,3-acyl transfer to generate an imide. The peptide imide can be hydrolysed to generate a native amide bond, or can undergo subsequent acyl transfer reactions to generate a range of modified peptides. Alternatively, the isoimide can be trapped by internal or external nucleophiles to generate a range of functionalised peptide motifs. We are investigating applications in peptide ligation, peptide residue excision/insertion, and peptide thioester synthesis.
Recent Publications
A. B. Taresh, C. A. Hutton, “Site Specific Preparation of N-Glycosylated Peptides: Thioamide-Directed Activation of Aspartate”, Angew. Chem. Int. Ed. 2022, 61, e202210367.
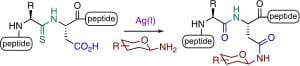
A. B. Taresh, C. A. Hutton, “Backbone thioamide directed macrocyclisation: lactam stapling of peptides”, Org. Biomol. Chem. 2022, 20, 1488–1492.
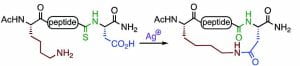
S. Shabani, C. A. Hutton, “Depsipeptide synthesis using a late-stage Ag(I)-promoted macrolactonisation of peptide thioamides, Chem. Commun. 2021, 57, 2081–2084.
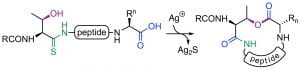
J. Shang, V. J. Thombare, C. L. Charron, U. Wille, C. A. Hutton, “Ring expansion of thiolactams via imide intermediates: an amino acid insertion strategy,” Chem. Eur. J. 2021, 26, 1620–1625.
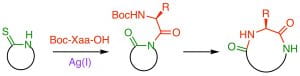
V. J. Thombare, C. A. Hutton, “Rapid, traceless, Ag(I)‐promoted macrocyclization of peptides possessing an N‐terminal thioamide,” Angew. Chem. Int. Ed., 2019, 58, 4998–5002.
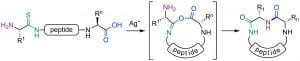
C. A. Hutton, J. Shang and U. Wille, “Synthesis of Peptides by Silver-Promoted Coupling of Carboxylates and Thioamides: Mechanistic Insight from Computational Studies,” Chem. Eur. J. 2016, 22, 3163–3169.
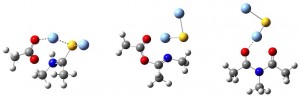
J. Shang, A. Pourvali, J. R. Cochrane and C. A. Hutton, “Steric and Electronic Effects in the Synthesis and Regioselective Hydrolysis of Unsymmetrical Imides,” Aust. J. Chem. 2015, 68, 1854–1858.
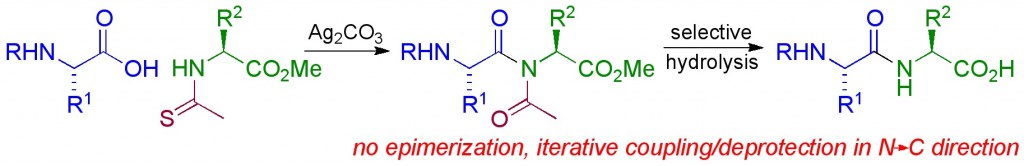
A. Pourvali, J. R. Cochrane, C. A. Hutton, “A new method for peptide synthesis in the N–C direction: amide assembly through silver-promoted reaction of thioamides,” Chem. Commun., 2014, 50, 15963–15966.