Organoboron Chemistry
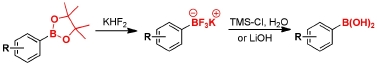
Our investigations both in the Petasis reaction and the use of tyrosine boronates had led to an interest in organoboron chemistry, particularly the efficient interconversion of boronate protecting groups. We have developed several methods for the cleavage of pinacol boronate esters to generate boronic acids under mild conditions.
Recent Publications
Q. I. Churches, C. A. Hutton, “Introduction, interconversion and removal of boron protecting groups,” in Boron Reagents in Synthesis. ACS Symposium Series, A. Coca, ed, American Chemical Society, 2016, vol 1236, Chapter 11, 357–377. ISBN13: 9780841231832, eISBN: 9780841231825.
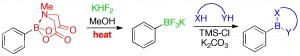
Q. I. Churches, J. F. Hooper, C. A. Hutton, “A General Method for Interconversion of Boronic Acid Protecting Groups: Trifluoroborates as Common Intermediates,” J. Org. Chem., 2015, 80, 5428–5435.
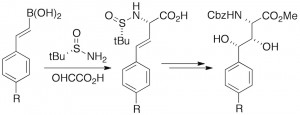
Q. I. Churches, J. M. White, C. A. Hutton, “Synthesis of β,γ-Dihydroxyhomotyrosines by a Tandem Petasis–Asymmetric Dihydroxylation Approach,” Org. Lett. 2011, 13, 2900–2903.
S. J. Shirbin, B. A. Boughton, S. C. Zammit, S. D. Zanatta, S. M. Marcuccio, C. A. Hutton, S. J. Williams, “Copper-free palladium-catalyzed Sonogashira and Hiyama cross-couplings using aryl imidazol-1-ylsulfonates,” Tetrahedron Lett., 2010, 51, 2971–2974.
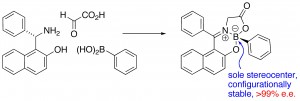
P. F. Kaiser, J. M. White and C. A. Hutton, “Enantioselective Preparation of a Stable Boronate Complex Stereogenic Only at Boron,” J. Am. Chem. Soc., 2008, 130, 16450–16451.